Human organotypic colon in vitro microtissue: unveiling a new window into colonic ...
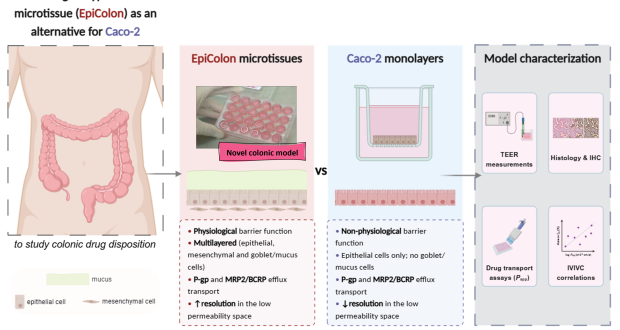
Read More »Colotan deals with a topic of growing medical and societal concern: obtaining a sufficiently high drug concentration in the colon (large intestine) to efficiently treat diseases of the large intestine. Oral administration of drugs is the most common delivery route due to its non-invasive character and convenience for the patient. However, issues related to pharmacokinetics (PK) and bioavailability remain a prominent cause of oral drug candidate attrition. Therefore, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) call for research into new predictive (virtual) tools, innovative drug delivery systems and precision medicine approaches. Oral formulations that release the drug in a pH- or time-dependent manner in the colon are commercially available. They are used to obtain topical treatment of colonic diseases such as inflammatory bowel diseases (IBD), that have become a global disease with high incidence in Europe, Oceania and the USA, but also for controlled release of a drug for systemic treatment (e.g. controlled release carvedilol). However, these formulations suffer from an inconsistent and inefficient performance, due to incomplete understanding of processes occurring in the colon. Moreover, the currently available evaluation tools to predict drug performance focus mainly on the upper GI tract, resulting in a lack of validated tools and models of the colon with high physiological relevance and predictive capability
Read more...
news
-
Pedro Canhão Costa Delivers a TED-Style Talk at World Organoid Research Day 2025 Among 60 Speakers
-
Alessia Favaron successfully defending her PhD on how JAK inhibitor drugs for inflammatory bowel disease (IBD) interact with the human gut microbiome
-
Outstanding cum laude! 1st COLOTAN PhD defence by Ines Pârente
-
A new COLOTAN publication that explores the potential of human intestinal organoids
Events
-
COLOTAN 4th Symposium
Check the programme of COLOTAN 4th symposium: Targeted Drug Delivery in the Gastrointestinal Tract Was held on 10th January 2024 in Cork, Ireland.
Read More » -
COLOTAN 4th Symposium – Targeted Drug Delivery in the Gastrointestinal tract
-
New Horizons in Drug Delivery and Formulation 2023
-
COLOTAN 3rd Symposium