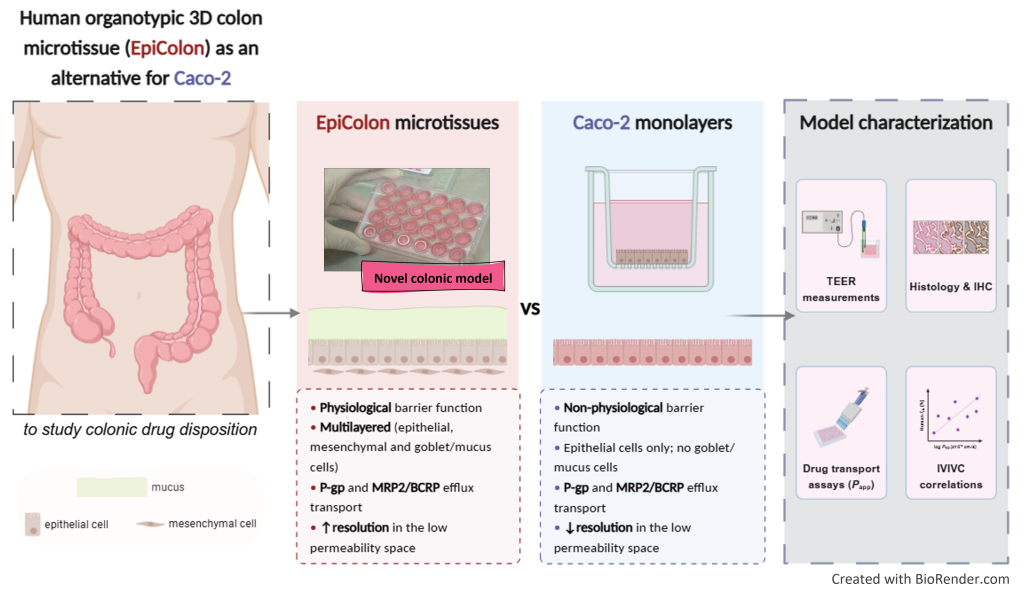
Human organotypic colon in vitro microtissue: unveiling a new window into colonic drug disposition
Pedro G.M. Canhão, Jan Snoeys, Suzy Geerinckx, Marjolein van Heerden, An Van den Bergh, Camden Holm, Jan Markus, Seyoum Ayehunie, Mario Monshouwer, Raymond Evers, Patrick Augustijns, Stephanie Kourula
Published in the European Journal of Pharmaceutical Sciences
Abstract
The purpose of this study was to evaluate EpiColon, a novel human organotypic 3D colon microtissue prototype, developed to assess colonic drug disposition, with a particular focus on permeability ranking, and compare its performance to Caco-2 monolayers.
EpiColon was characterized for barrier function using transepithelial electrical resistance (TEER), morphology via histology and immunohistochemistry, and functionality through drug transport studies measuring apparent permeability (Papp). Cutoff thresholds for the permeability of FITC–dextran 4 kDa (FD4), FITC–dextran 10 kDa (FD10S), and [14C]mannitol were established to monitor microtissue integrity. Permeability of EpiColon for 20 benchmark drugs was compared with Caco-2 data, and the activity of pivotal efflux transporters, including multidrug resistance protein 1/P-glycoprotein (MDR1/P-gp), along with multidrug resistance protein 2 (MRP2) and breast cancer resistance protein (BCRP), was evaluated using selective substrates.
EpiColon exhibited a physiological barrier function (272.0 ± 53.05 Ω x cm2) and effectively discriminated between high (e.g., budesonide and [3H]metoprolol) and low permeable compounds (e.g., [3H]atenolol and [14C]mannitol). The model demonstrated functional activity for key efflux transporters, with efflux ratios of 2.32 for [3H]digoxin (MDR1/P-gp) and 3.34 for sulfasalazine (MRP2 and BCRP). Notably, EpiColon showed an enhanced dynamic range in the low permeability range, differentiating Papp between FD4 and FD10S, in contrast to Caco-2 monolayers. Significant positive correlations were observed between human fraction absorbed (fabs) and logarithmically transformed Papp [AP-BL] values for both EpiColon (rs = 0.68) and Caco-2 (rs = 0.68). Furthermore, EpiColon recapitulates some essential phenotypic and cellular features of the human colon, including the expression of critical marker genes (Pan-Cytokeratin+: epithelial/colonocytes, Vimentin+: mesenchymal/fibroblast, and Alcian Blue+: goblet cell/mucus).
In conclusion, EpiColon is a promising platform that offers a valuable complement to conventional Caco-2 monolayers for studying colonic drug disposition. However, the presence of flat and some cuboidal cells, along with low throughput, must be addressed to improve its applicability in both academic research and pharmaceutical industry.
🔗 Read the full paper here & share it.
Key Highlights:
⭐ EpiColon mimics structural and cellular features of the human colon.
⭐ EpiColon demonstrates TEER akin to native human colonic tissue.
⭐ EpiColon discriminates between high and low permeable drugs.
⭐ EpiColon may help rank small molecules in the low permeability category.
Why It Matters:
⭐ Provides a physiologically relevant alternative to Caco-2 with improved structural and functional attributes.
⭐ Aligns with the 3Rs by reducing dependence on animal models.
This work stems from an ongoing 4-year collaboration between Preclinical Sciences & Translational Safety at Johnson & Johnson and MatTek Life Sciences.
💭 Engage with us on LinkedIn: How might complex in vitro models like EpiColon influence pharmacokinetic evaluations?