Physiologically based biopharmaceutics modeling of regional and colon absorption in humans
C. Tannergren, H. Jadhav, E. Eckernäs, J. Fagerberg, P. Augustijns, E. Sjögren
Abstract
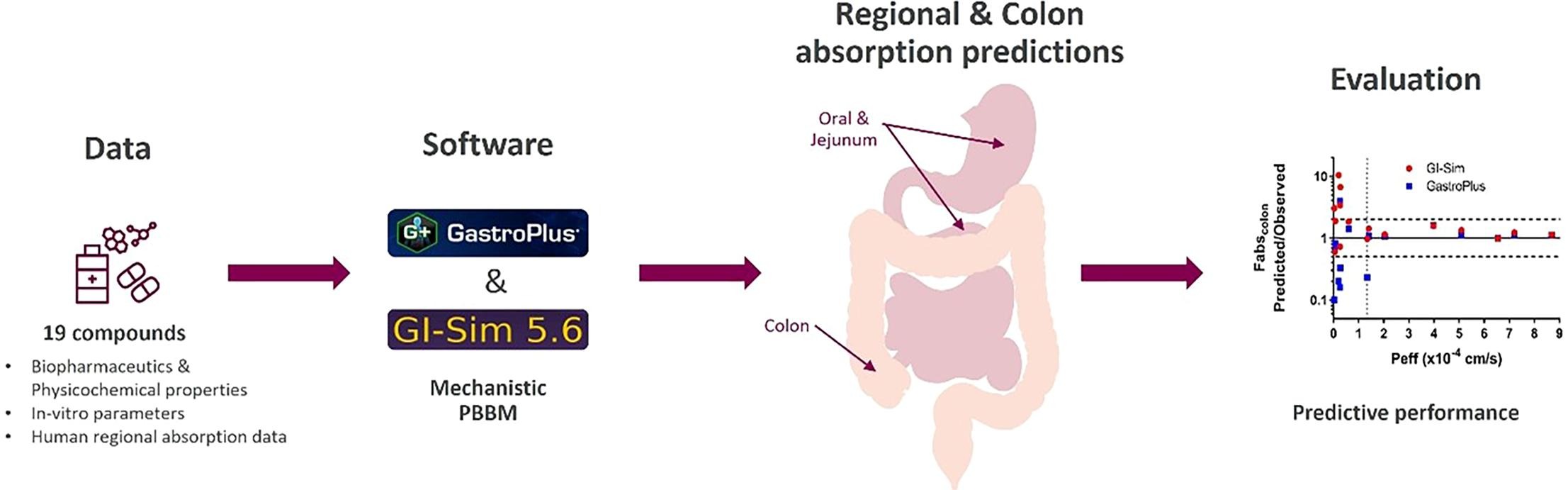
Colon absorption is a key determinant for successful development of extended release and colon targeted drug products. This is the first systematic evaluation of the ability to predict in vivo regional differences in absorption and the extent of colon absorption in humans using mechanistic physiologically based biopharmaceutics modeling (PBBM). A new dataset, consisting of 19 drugs with a wide range of biopharmaceutics properties and extent of colon absorption in humans, was established. Mechanistic predictions of the extent of absorption and plasma exposure after oral, or jejunal and direct colon administration were performed in GastroPlus and GI-Sim using an a priori approach. Two new colon models developed in GI-Sim, were also evaluated to assess if the prediction performance could be improved. Both GastroPlus and GI-Sim met the pre-defined criteria for accurate predictions of regional and colon absorption for high permeability drugs irrespective of formulation type, while the prediction performance was poor for low permeability drugs. For solutions, the two new GI-Sim colon models improved the colon absorption prediction performance for the low permeability drugs while maintaining the accurate prediction performance for the high permeability drugs. In contrast, the prediction performance decreased for non-solutions using the two new colon models. In conclusion, PBBM can be used with sufficient accuracy to predict regional and colon absorption in humans for high permeability drugs in candidate selection as well as early design and development of extended release or colon targeted drug products. The prediction performance of the current models needs to be improved to allow high accuracy predictions for commercial drug product applications including highly accurate predictions of the entire plasma concentration–time profiles as well as for low permeability drugs.
Read the full article here: https://doi.org/10.1016/j.ejpb.2023.03.013