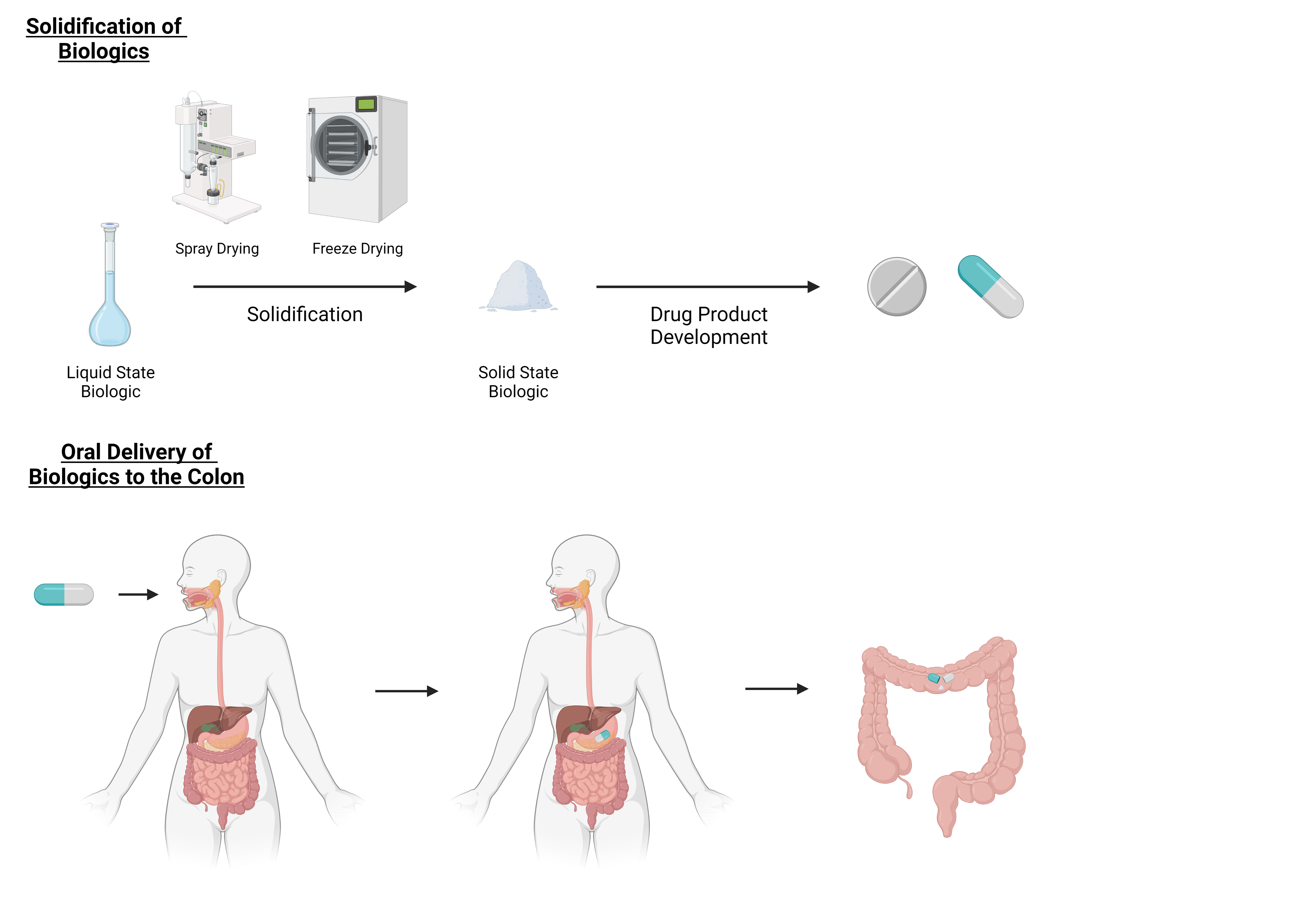
Solidification and oral delivery of biologics to the colon – A review by COLOTAN researcher Katharina Kopp with the supervision of Lien Saerens (Eurofins), Jody Voorspoels (Eurofins) and Guy Van den Mooter (KU Leuven) that has been published recently in the European Journal of Pharmaceutical Sciences.
Abstract
The oral delivery of biologics such as therapeutic proteins, peptides and oligonucleotides for the treatment of colon related diseases has been the focus of increasing attention over the last years. However, the major disadvantage of these macromolecules is their degradation propensity in liquid state which can lead to the undesirable and complete loss of function. Therefore, to increase the stability of the biologic and reduce their degradation propensity, formulation techniques such as solidification can be performed to obtain a stable solid dosage form for oral administration. Due to their fragility, stress exerted on the biologic during solidification has to be reduced with the incorporation of stabilizing excipients into the formulation. This review focuses on the state-of-the-art solidification techniques required to obtain a solid dosage form for the oral delivery of biologics to the colon and the use of suitable excipients for adequate stabilization upon solidification. The solidifying processes discussed within this review are spray drying, freeze drying, bead coating and also other techniques such as spray freeze drying, electro spraying, vacuum- and supercritical fluid drying. Further, the colon as site of absorption in both healthy and diseased state is critically reviewed and possible oral delivery systems for biologics are discussed.
Read the full article here.